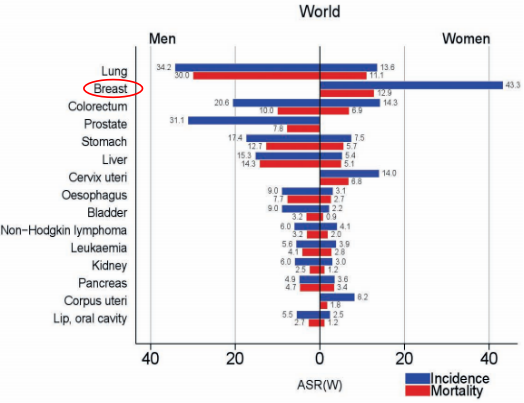
The latest global cancer statistics from the World Health Organization’s International Agency for Research on Cancer (IARC) in 2020 marked a significant milestone: breast cancer cases, predominantly in women, surpassed lung cancer in the global count for the first time, registering 2.26 million new cases. This alarming rise places a spotlight on the critical role of advanced diagnostic techniques.
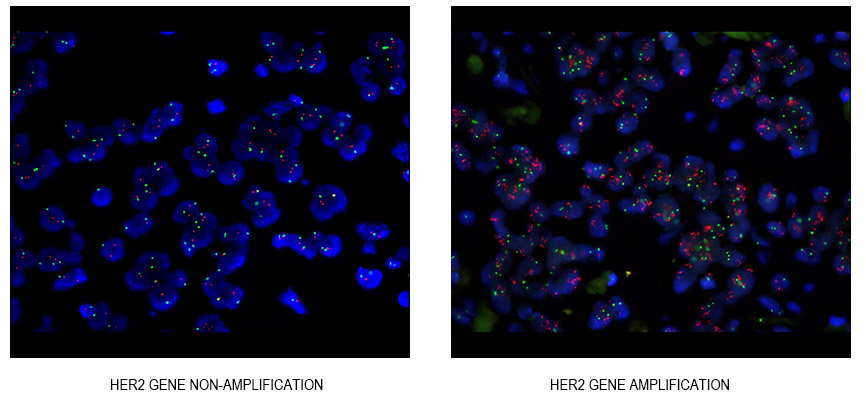
Among these, the Human Epidermal growth factor Receptor-2 (HER-2), belonging to the EGFR family, stands out. About a quarter of breast cancer patients exhibit amplification of the HER-2 gene, which is intricately linked to the aggressive nature of many epithelial cell cancers, characterized by robust metastatic capabilities, reduced responsiveness to chemotherapy, and a higher likelihood of recurrence. This gene amplification predominantly occurs on the long arm of chromosome 17 and can also transpose to other chromosomes. In situ amplification, particularly stable in chromosome structures like centromeres and adjacent genes, is a noteworthy aspect.
The conventional method of detecting HER-2 gene amplification is through Fluorescence in situ Hybridization (FISH) technology. FISH, regarded as the gold standard, allows direct observation of the amplification process. The “Breast Cancer HER-2 Testing Guidelines (2019 Edition)” emphasizes the importance of HER-2 copy number in determining gene amplification.
However, FISH has limitations, including challenges in addressing tumor heterogeneity, labor-intensive staining, and preparation processes, high costs, and dependence on specialized laboratory expertise. These constraints have paved the way for digital PCR (dPCR) technology. dPCR’s ability to perform absolute quantitative detection makes it uniquely advantageous in detecting HER-2 gene copy number variations. It allows for the direct detection of HER-2 gene amplification from peripheral blood cell-free DNA samples, mitigating the discomfort and risks associated with tissue biopsies. The specificity of dPCR highlights its potential in targeted treatment strategies for cancer recurrence and metastasis.
Unicorn, leveraging the AccuONE digital PCR platform, developed a comprehensive HER-2 gene digital PCR detection kit, leveraging the AccuONE digital PCR platform. The kit’s design principles underscore that for samples with uniform cell properties, the ideal HER-2/CEP17 ratio, as determined by dPCR, should be a natural number, reflecting the typical chromosomal distribution. For instance, normal human tissue should show a HER-2/CEP17 value of 1. Unlike FISH, dPCR’s threshold for determining HER-2 gene amplification is more stringent, offering a narrower range for amplification identification and thus enhancing precision in sample analysis.
The development of this kit also accounted for potential missed detections in cases of HER2 amplification, using an internal reference gene to mitigate the effects of amplification phenomena in the centromere region of chromosome 17. This consideration is vital in ensuring the accuracy of HER2 copy number variation detection.
In conclusion, the clinical application of Trastuzumab (Herceptin) for breast cancer patients with HER-2 amplification-positive tumors has significantly improved patient outcomes. This underscores the importance of precise HER-2 gene amplification detection in clinical decision-making and patient management. For a more comprehensive understanding of HER-2 digital PCR detection and its applications, resources such as the “Fifth PCR Technology Network Conference” offer valuable insights, particularly in sessions focusing on the application of multi-color fluorescence digital PCR technology in HER2 copy number variation detection.
You can read the article The Importance of Genetic Screening for Breast Cancer Patients.
Here is some further educational files for you to learn more about HER2 detection in digital PCR technology



