Pfizer, Moderna’s COVID-19 vaccine and the latest Janssen single-dose jab have turned the vaccine cold chain into hot topics. But what is a cold chain? How does it work?
What is Cold Chain?
General speaking, a cold chain is a temperature-controlled supply chain, in order to maintain quality and safety of the products from the point of origin through the distribution chain to the final destination/customer. It is used to preserve and to extend and ensure the shelf life of products, such as fresh agricultural produce, seafood, frozen food, chemicals and pharmaceutical products.
What is Vaccine Cold Chain?
Furthermore, the vaccine cold chain is a set of strict rules and procedures that ensure the proper storage and distribution of vaccines from the manufacturer to the service provider even to the vaccine receiver. This process is interconnected with refrigeration and freezer equipment that allows vaccines to be stored at recommended temperatures to maintain their potency and effectiveness.
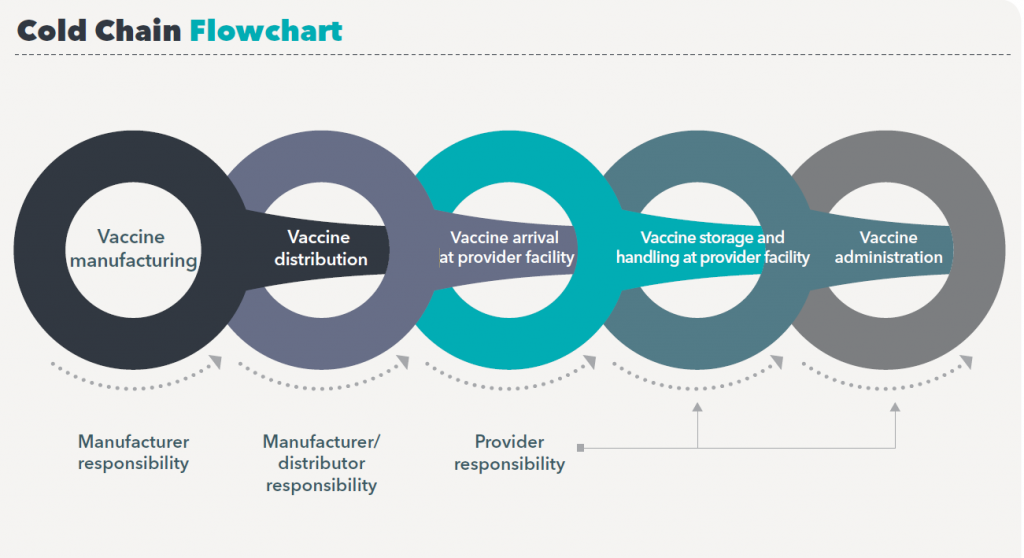
Due to the emergence of COVID-19 pandemic since 2019, COVID-19 vaccine management and distribution is a new and complex challenge that involves with multiple stakeholders in managing a global network of cold rooms, freezers, refrigerators and cold boxes and other carriers in order to keep to recommended temperature. Below illustrates a process of how a vaccine finally gets to its destination – the vaccine receivers.

What is a Cold Chain Breach?
A cold chain breach occurs when vaccine storage temperatures goes outside of the recommended range of +2 °C to+8°C, which is provided by 2-8℃ pharmacy refrigerators. If there is a cold chain breach, should be reported immediately to related department.
Principles of Safe Vaccine Storage Management
Vaccines are sensitive biological products that may become less effective or destroyed when expose to temperatures outside the recommended range. If an ineffective product is used, recipients may not be protected against vaccine preventable diseases and this may result in re-emergence or occurrence of those diseases, and also re-vaccination is required occurring financially lost to the service provider.
There are three key elements combine to a proper vaccine cold chain for vaccine transport, storage, and handling:
- Trained staff
- Proper transportation and storage equipment
- Efficient vaccine management procedures
Trained Staff
All staff members who is responsible for handling and administrating vaccines should be trained in proper vaccine storage and handling practices, they need to be aware of their responsibilities relating to the cold chain, and understand the importance of effective vaccine management. According to CDC, Staff who monitor and record temperatures of vaccine storage units should emergency protocols. CDC also recommends to develop and maintain clearly written, detailed, and up-to-date storage and handling standard operating procedures (SOPs). This should contain at least three major areas:
- General information – this includes general information about staff and related stakeholders , which may include: vaccine manufacturers, service providers, own staff contact details and job description; regular forms and stickers, training materials etc.
- Routine storage and handling SOPs – include information for all aspects of vaccine inventory management, from ordering to monitoring storage conditions.
- Emergency SOPs – define procedures to be taken in the event of equipment malfunctions, power failures, natural disasters, or other emergencies that might compromise vaccine storage conditions.
Proper Transportation and Storage Equipment
It is important to have proper storage and monitoring equipment right from the manufacturer till medical facilities, that is been set up correctly, maintained properly and repaired accordingly to its needs. Different COVID-19 vaccines have different types of storage requirement in relation to the types of vaccine storage units and temperature monitoring devices used to maintain the cold chain, including the use of ultra-cold units. Temperature ranges for COVID-19 vaccines are the main aspect in determine which storage devices to use as different storage unit offers different cooling range.
- It is recommended by CDC to use purpose-built or pharmaceutical-grade units designed for refrigeration or freezing biological material such as vaccines. These units can be compact, under-the-counter style or large units.
- Every vaccine storage unit must have a Temperature Monitoring Device (TMD). In this case, TMD can be as a thermometer or a digital data logger. It is recommended to use a digital IoT data logger that can remotely record and transfer data or send alarm to users when necessary.
Efficient Vaccine Management Procedures
In General, effectively management of vaccine cold chain is not only using purpose-built units for the desired temperature control, but also involves using correct devices and procedures during every stage of cold chain which includes delivery, storage and transportation process.
Unicorn is a leading medical and laboratory company with decades of experience in R&D and manufacturing, who is able to provide you with comprehensive solution for your vaccine cold chain management no better the part of the cold chain business you are in. Contact us today and get a free consultation for effective vaccine cold chain management solution.